Cloning and characterization of the CYS3 (CYI1) gene of Saccharomyces cerevisiae.
B Ono, K Tanaka, K Naito, C Heike, S Shinoda, S Yamamoto, S Ohmori, T Oshima, A Toh-e
文献索引:J. Bacteriol. 174(10) , 3339-47, (1992)
全文:HTML全文
摘要
A DNA fragment containing the Saccharomyces cerevisiae CYS3 (CYI1) gene was cloned. The clone had a single open reading frame of 1,182 bp (394 amino acid residues). By comparison of the deduced amino acid sequence with the N-terminal amino acid sequence of cystathionine gamma-lyase, CYS3 (CYI1) was concluded to be the structural gene for this enzyme. In addition, the deduced sequence showed homology with the following enzymes: rat cystathionine gamma-lyase (41%), Escherichia coli cystathionine gamma-synthase (36%), and cystathionine beta-lyase (25%). The N-terminal half of it was homologous (39%) with the N-terminal half of S. cerevisiae O-acetylserine and O-acetylhomoserine sulfhydrylase. The cloned CYS3 (CYI1) gene marginally complemented the E. coli metB mutation (cystathionine gamma-synthase deficiency) and conferred cystathionine gamma-synthase activity as well as cystathionine gamma-lyase activity to E. coli; cystathionine gamma-synthase activity was detected when O-succinylhomoserine but not O-acetylhomoserine was used as substrate. We therefore conclude that S. cerevisiae cystathionine gamma-lyase and E. coli cystathionine gamma-synthase are homologous in both structure and in vitro function and propose that their different in vivo functions are due to the unavailability of O-succinylhomoserine in S. cerevisiae and the scarceness of cystathionine in E. coli.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
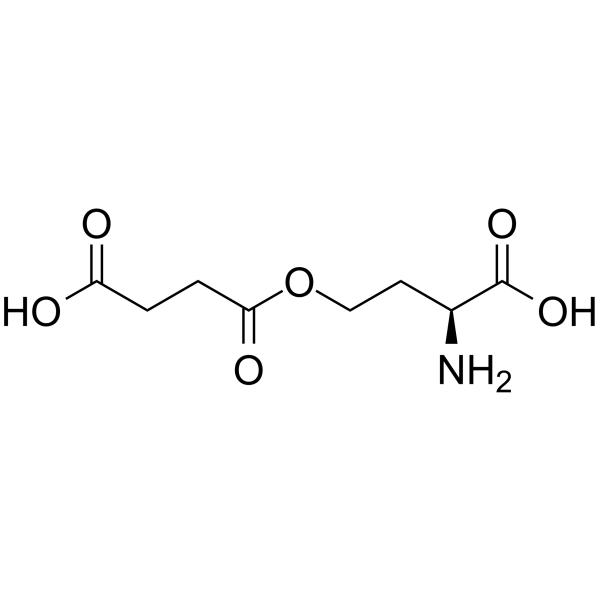 |
O-琥珀酰-L-高丝氨酸
CAS:1492-23-5 |
C8H13NO6 |
|
The enzymology of cystathionine biosynthesis: strategies for...
2005-01-01 [Arch. Biochem. Biophys. 433 , 166-175, (2005)] |
|
Cloning and characterization of two Lactobacillus casei gene...
2008-01-01 [Appl. Environ. Microbiol. 74 , 99-106, (2008)] |
|
Enzymatic characterization and inhibitor discovery of a new ...
2008-01-01 [J. Biochem. 143 , 59-68, (2008)] |
|
Pathways of assimilative sulfur metabolism in Pseudomonas pu...
1999-09-01 [J. Bacteriol. 181(18) , 5833-7, (1999)] |
|
A direct sulfhydrylation pathway is used for methionine bios...
1995-02-01 [Microbiology 141 ( Pt 2) , 431-9, (1995)] |