Evidence for dynamics in proteins as a mechanism for ligand dissociation.
Mary J Carroll, Randall V Mauldin, Anna V Gromova, Scott F Singleton, Edward J Collins, Andrew L Lee
文献索引:Nat. Chem. Biol. 8(3) , 246-52, (2012)
全文:HTML全文
摘要
Signal transduction, regulatory processes and pharmaceutical responses are highly dependent upon ligand residence times. Gaining insight into how physical factors influence residence times (1/k(off)) should enhance our ability to manipulate biological interactions. We report experiments that yield structural insight into k(off) involving a series of eight 2,4-diaminopyrimidine inhibitors of dihydrofolate reductase whose binding affinities vary by six orders of magnitude. NMR relaxation-dispersion experiments revealed a common set of residues near the binding site that undergo a concerted millisecond-timescale switching event to a previously unidentified conformation. The rate of switching from ground to excited conformations correlates exponentially with the binding affinity K(i) and k(off), suggesting that protein dynamics serves as a mechanical initiator of ligand dissociation within this series and potentially for other macromolecule-ligand systems. Although the forward rate of conformational exchange, k(conf,forward), is faster than k(off), the use of the ligand series allowed for connections to be drawn between kinetic events on different timescales.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
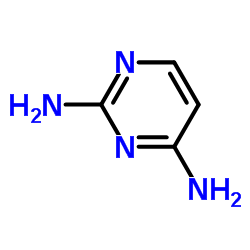 |
2,4-二氨基嘧啶
CAS:156-81-0 |
C4H6N4 |
|
Contribution of nitric oxide-dependent guanylate cyclase and...
2015-12-01 [Neuropharmacology 99 , 422-31, (2015)] |
|
Design and optimization of hybrid of 2,4-diaminopyrimidine a...
2015-01-01 [Eur. J. Med. Chem. 96 , 269-80, (2015)] |
|
Cocrystals of 6-methyl-2-thiouracil: presence of the accepto...
2015-03-01 [Acta Crystallogr. C Struct. Chem. 71(Pt 3) , 229-38, (2015)] |
|
Ultra performance liquid chromatography tandem mass spectrom...
2009-03-01 [Anal. Bioanal. Chem 393(6-7) , 1709-18, (2009)] |
|
Inhibitory activities of three classes of acyclic nucleoside...
2007-06-01 [Antimicrob. Agents Chemother. 51(6) , 2268-73, (2007)] |