Radical-mediated nitrile translocation as the key step in the stereoselective transformation of 2-(4-chloro-2-cyanobutyl)aziridines to methyl cis-(1-arylmethyl-4-phenylpiperidin-2-yl)acetates.
Karel Vervisch, Matthias D'hooghe, Karl W Törnroos, Norbert De Kimpe
文献索引:Org. Biomol. Chem. 10(16) , 3308-14, (2012)
全文:HTML全文
摘要
Non-activated 2-(4-chloro-2-cyano-2-phenylbutyl)aziridines were used as building blocks for the stereoselective synthesis of novel cis-2-cyanomethyl-4-phenylpiperidines via a microwave-assisted aziridine to piperidine ring expansion followed by a radical-induced nitrile translocation through initial formation and subsequent cleavage of intermediate bicyclic iminyl radicals. Furthermore, these 2-(cyanomethyl)piperidines were shown to be eligible substrates for the preparation of methyl cis-(1-arylmethyl-4-piperidin-2-yl)acetates through a Pinner reaction using gaseous HCl in methanol.This journal is © The Royal Society of Chemistry 2012
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
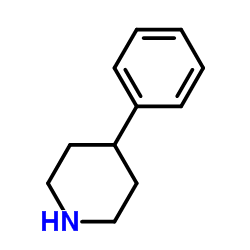 |
4-苯基哌啶
CAS:771-99-3 |
C11H15N |
|
A strategy for isotope containment during radiosynthesis--de...
2008-11-21 [Org. Biomol. Chem. 6 , 4093-4095, (2008)] |
|
Structure-activity studies of morphine fragments. I. 4-alkyl...
1988-09-01 [Mol. Pharmacol. 34(3) , 363-76, (1988)] |
|
Clandestine drug synthesis.
1986-01-01 [Med. Res. Rev. 6(1) , 41-74, (1986)] |
|
Novel 1-phenylpiperazine and 4-phenylpiperidine derivatives ...
1991-12-01 [J. Med. Chem. 34(12) , 3360-5, (1991)] |
|
Influence of cocaine history on the behavioral effects of Do...
[Biol. Psychiatry 36(5) , 1104-13, (2011)] |