Organic & Biomolecular Chemistry
2008-11-21
A strategy for isotope containment during radiosynthesis--devolatilisation of bromobenzene by fluorous-tagging-Ir-catalysed borylation en route to the 4-phenylpiperidine pharmacophore.
Alan C Spivey, Laetitia J Martin, Chih-Chung Tseng, George J Ellames, Andrew D Kohler
文献索引:Org. Biomol. Chem. 6 , 4093-4095, (2008)
全文:HTML全文
摘要
Syntheses of two 4-phenylpiperidines from bromobenzene have been developed involving anchoring to a fluorous-tag, Ir-catalysed borylation, Pd- and Co-catalysed elaboration then traceless cleavage. Although performed using 'cold' (i.e. unlabelled) bromobenzene as the starting material, these routes have been designed to minimise material loss via volatile intermediates and expedite purification during radiosynthesis from 'hot' (i.e. [(14)C] labelled) bromobenzene.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
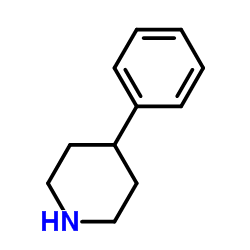 |
4-苯基哌啶
CAS:771-99-3 |
C11H15N |
相关文献:
更多...
|
Structure-activity studies of morphine fragments. I. 4-alkyl...
1988-09-01 [Mol. Pharmacol. 34(3) , 363-76, (1988)] |
|
Radical-mediated nitrile translocation as the key step in th...
2012-04-28 [Org. Biomol. Chem. 10(16) , 3308-14, (2012)] |
|
Clandestine drug synthesis.
1986-01-01 [Med. Res. Rev. 6(1) , 41-74, (1986)] |
|
Novel 1-phenylpiperazine and 4-phenylpiperidine derivatives ...
1991-12-01 [J. Med. Chem. 34(12) , 3360-5, (1991)] |
|
Influence of cocaine history on the behavioral effects of Do...
[Biol. Psychiatry 36(5) , 1104-13, (2011)] |