| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
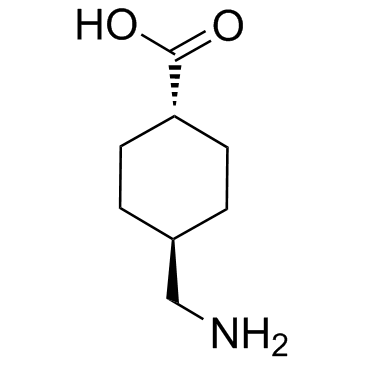 |
凝血酸,氨甲环酸
CAS:1197-18-8 |
|
 |
纤溶酶原 来源于人类血浆
CAS:9001-91-6 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
凝血酸,氨甲环酸
CAS:1197-18-8 |
|
 |
纤溶酶原 来源于人类血浆
CAS:9001-91-6 |