| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
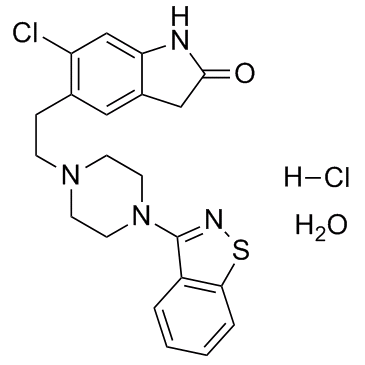 |
盐酸齐拉西酮
CAS:138982-67-9 |
|
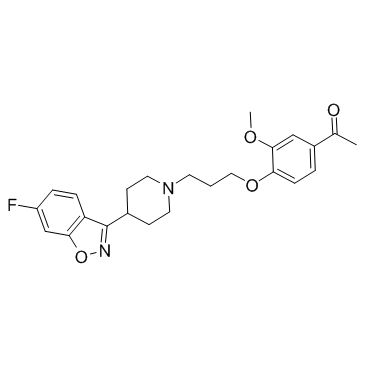 |
伊潘立酮
CAS:133454-47-4 |
|
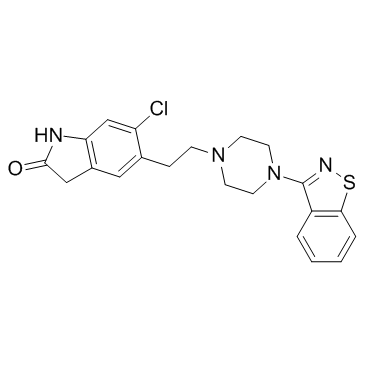 |
齐拉西酮
CAS:146939-27-7 |