Synthesis of Amphiphilic Block Copolypept(o)ides by Bifunctional Initiators: Making PeptoMicelles Redox Sensitive.
Regina Holm, Kristina Klinker, Benjamin Weber, Matthias Barz
文献索引:Macromol. Rapid Commun. 36 , 2083-91, (2016)
全文:HTML全文
摘要
In this work, the synthesis of polypeptoid-block-polypeptide copolymers (block copolypept(o)ides) based on bifunctional initiators is described, which introduces a distinct chemical entity at the connection between both blocks. With a view towards redox-sensitive block copolypept(o)ides, a cystamine-based initiator was used to synthesize polysarcosine macroinitiators with degrees of polymerization (Xn) between 100 and 200 displaying monomodal molecular weight distributions and dispersities (Đ) around 1.1 as determined by size exclusion chromatography. Block copolypept(o)ides with a poly(γ-t-butyloxycarbonyl-L-glutamate) (PGlu(O(t) Bu)) block (Xn = 25 or 50) were synthesized by controlled N-carboxyanhydride polymerization. Resulting block copolymers possess monomodal molecular weight distributions, dispersities around 1.2 and were applied to degradation studies. While block copolypept(o)ides are stable at 10 × 10(-6) M, they degrade over time at GSH concentrations of 10 × 10(-3) and 100 × 10(-3) M. Furthermore, these disulfide-containing block copolymers form PeptoMicelles, which degrade at intracellular GSH concentrations while remaining stable at extracellular GSH levels.© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
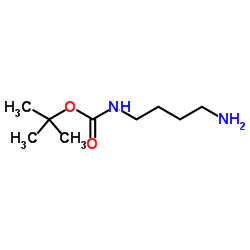 |
N-(叔丁氧羰基)-1,4-丁二胺
CAS:68076-36-8 |
C9H20N2O2 |
|
Peptide splicing in a double-sequence analogue of trypsin in...
2015-05-01 [Biopolymers 104 , 206-12, (2015)] |
|
Synthesis, tubulin binding, antineoplastic evaluation, and s...
1989-02-01 [J. Med. Chem. 32 , 409, (1989)] |
|
Synthesis and biological evaluation of new citrate-based sid...
2002-05-09 [J. Med. Chem. 45 , 2056, (2002)] |
|
18F-labeled insulin: a prosthetic group methodology for inco...
1989-05-30 [Biochemistry 28 , 4801, (1989)] |
|
Rationally designed peptoids modulate aggregation of amyloid...
2014-07-16 [ACS Chem. Neurosci. 5(7) , 552-8, (2014)] |