Synthesis, tubulin binding, antineoplastic evaluation, and structure-activity relationship of oncodazole analogues.
L I Kruse, D L Ladd, P B Harrsch, F L McCabe, S M Mong, L Faucette, R Johnson
文献索引:J. Med. Chem. 32 , 409, (1989)
全文:HTML全文
摘要
In an attempt to identify a soluble oncodazole analogue that could be easily formulated, a series of substituted oncodazoles was synthesized and evaluated for tubulin binding affinity, in vitro cytotoxicity against cultured mouse B-16 cells, and ability to prolong lifespan at the maximally tolerated dose in the P388 mouse leukemia model. Biological evaluation of all the isomeric methyloncodazoles demonstrated the thiophene 4'-position to be the only site of significant bulk tolerance, although substitution of this position with polar or charged functional groups abolished biological activity. Simple esters of the 4'-carboxymethyloncodazole were shown to have enhanced antitumor activity and tubulin binding affinity relative to oncodazole. Despite a failure of this study to identify a water-soluble oncodazole with antitumor activity, the structure-activity relationship developed led to a derivative with enhanced activity in the P388 leukemia model and facilitated the preparation of a biologically active photolabile analogue.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
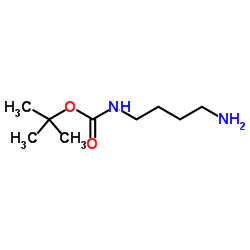 |
N-(叔丁氧羰基)-1,4-丁二胺
CAS:68076-36-8 |
C9H20N2O2 |
|
Peptide splicing in a double-sequence analogue of trypsin in...
2015-05-01 [Biopolymers 104 , 206-12, (2015)] |
|
Synthesis and biological evaluation of new citrate-based sid...
2002-05-09 [J. Med. Chem. 45 , 2056, (2002)] |
|
18F-labeled insulin: a prosthetic group methodology for inco...
1989-05-30 [Biochemistry 28 , 4801, (1989)] |
|
Rationally designed peptoids modulate aggregation of amyloid...
2014-07-16 [ACS Chem. Neurosci. 5(7) , 552-8, (2014)] |
|
Synthesis of Amphiphilic Block Copolypept(o)ides by Bifuncti...
2015-12-01 [Macromol. Rapid Commun. 36 , 2083-91, (2016)] |