The preparation and bioactivities of (-)-isovelleral.
M Jonassohn, R Hjertberg, H Anke, K Dekermendjian, A Szallasi, E Thines, R Witt, O Sterner
文献索引:Bioorg. Med. Chem. 5(7) , 1363-7, (1997)
全文:HTML全文
摘要
The resolution of synthetic (+/-)-isovelleral (1), via chromatographic separation of the two diastereomers of the (-)-menthoxyacetic acid diester of the corresponding (+/-)-diol (3), yielded both enantiomers of the bioactive fungal metabolite (+)-isovelleral (1). While the antimicrobial and cytotoxic activities of the two enantiomers are comparable, natural (+)-1 is approximately 10 times more mutagenic towards Ames' tester strain TA98 than (-)-1. The two enantiomers of the cyclopropane ring isomer 2 also possess negligible mutagenicity compared to (+)-1. Both (+)-1 and (-)-1 have the same affinity for the vanilloid receptor, but significant different affinity for the dopamine D1 receptor.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
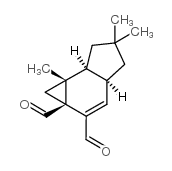 |
异绒白乳菇醛
CAS:37841-91-1 |
C15H20O2 |
|
The stimulation of capsaicin-sensitive neurones in a vanillo...
1996-09-01 [Br. J. Pharmacol. 119 , 283-290, (1996)] |
|
Structure-activity relationships for unsaturated dialdehydes...
1995-07-01 [Acta Chem. Scand. 49(7) , 530-5, (1995)] |
|
Sesquiterpenoid unsaturated dialdehydes increase the concent...
1994-01-01 [Nat. Toxins 2(2) , 89-95, (1994)] |
|
A novel route to the marasmane skeleton via a tandem rearran...
2001-04-06 [J. Org. Chem. 66(7) , 2350-7, (2001)] |
|
Characterization using FLIPR of rat vanilloid receptor (rVR1...
2000-06-01 [Br. J. Pharmacol. 130(4) , 916-22, (2000)] |