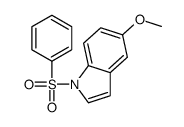
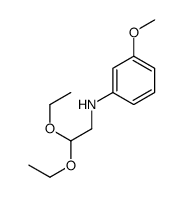
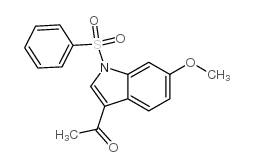
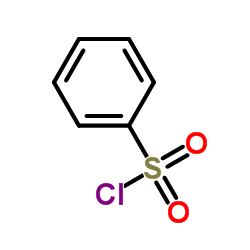
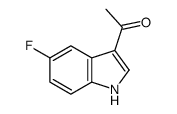
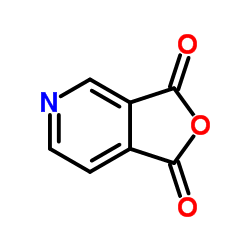
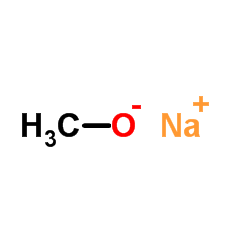
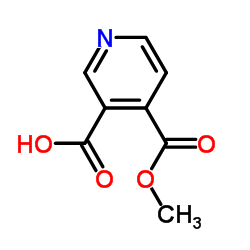
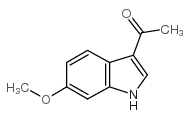
|
~82% |
|
~94% |
|
~% |
|
~% |
|
~98% |
|
~% |
|
~% |
|
~% |
|
~35% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~73% |
|
~76% |
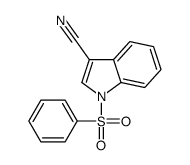


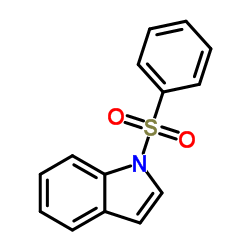

![1-[1-(benzenesulfonyl)indol-3-yl]ethanone结构式](https://image.chemsrc.com/caspic/040/99532-45-3.png)
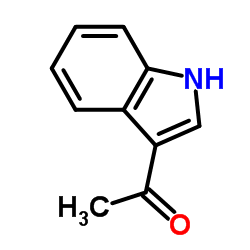
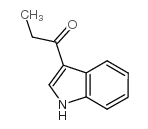
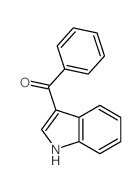

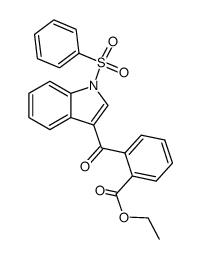
![6,11-Dihydro-5H-benzo[b]carbazole-6,11-dione结构式](https://image.chemsrc.com/caspic/243/6451-05-4.png)