| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
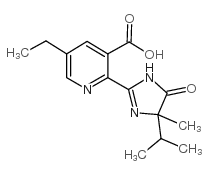 |
咪草烟
CAS:81335-77-5 |
|
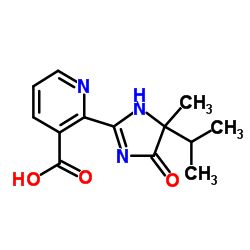 |
灭草烟
CAS:81334-34-1 |
|
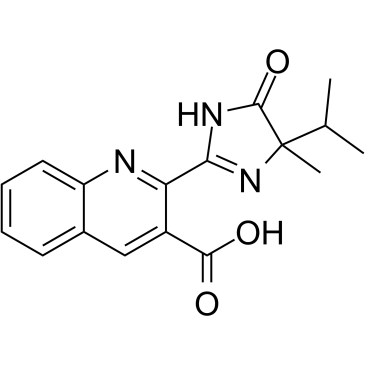 |
灭草喹
CAS:81335-37-7 |