Photodegradation of the herbicide imazethapyr in aqueous solution: effects of wavelength, pH, and natural organic matter (NOM) and analysis of photoproducts.
Ryan Espy, Emily Pelton, Annie Opseth, Jonathan Kasprisin, Amanda M Nienow
文献索引:J. Agric. Food Chem. 59(13) , 7277-85, (2011)
全文:HTML全文
摘要
The photodegradation of imazethapyr, 5-ethyl-2-(4-isopropyl-4-methyl-5-oxo-4,5-dihydroimidazol-1H-3-yl)nicotinic acid, has been investigated in phosphate buffers and in buffered solutions containing natural organic matter (NOM). Imazethapyr degrades most quickly under 253.7 nm light and at pH values >4. The presence of NOM in solution caused the reaction rate constants for the photodegradation to decrease, with higher concentrations of NOM having a larger effect. Calculations suggest light screening is the major effect of the NOM. Seven photoproducts have been identified, and a photodegradation mechanism is proposed.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
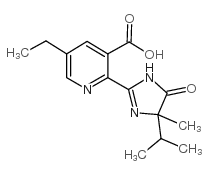 |
咪草烟
CAS:81335-77-5 |
C15H19N3O3 |
|
Enantioselective phytotoxicity of the herbicide imazethapyr ...
2011-08-15 [Environ. Sci. Technol. 45(16) , 7036-43, (2011)] |
|
Molecular mechanism of enantioselective inhibition of acetol...
2010-04-14 [J. Agric. Food Chem. 58(7) , 4202-6, (2010)] |
|
Enantioselective phytotoxicity of the herbicide imazethapyr ...
2011-01-01 [PLoS ONE 6(5) , e19451, (2011)] |
|
Abiotic degradation (photodegradation and hydrolysis) of imi...
2008-02-01 [J. Environ. Sci. Health B 43(2) , 105-12, (2008)] |
|
Unraveling the role of fermentation in the mode of action of...
2011-09-01 [J. Plant Physiol. 168(13) , 1568-75, (2011)] |