| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
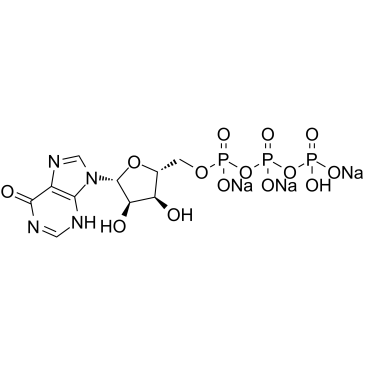 |
肌苷-5'-三磷酸三钠盐
CAS:35908-31-7 |
|
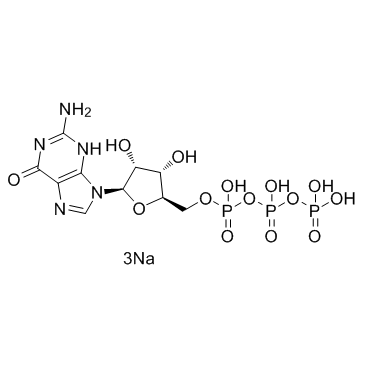 |
鸟苷-5'-三磷酸钠盐(GTP)
CAS:36051-31-7 |