Mapping interactions between the Ca2+-ATPase and its substrate ATP with infrared spectroscopy.
Man Liu, Andreas Barth
文献索引:J. Biol. Chem. 278 , 10112-10118, (2003)
全文:HTML全文
摘要
Infrared spectroscopy has been used to map substrate-protein interactions: the conformational changes of the sarcoplasmic reticulum Ca(2+)-ATPase upon nucleotide binding and ATPase phosphorylation were monitored using the substrate ATP and ATP analogues (2'-deoxy-ATP, 3'-deoxy-ATP, and inosine 5'-triphosphate), which were modified at specific functional groups of the substrate. Modifications to the 2'-OH, the 3'-OH, and the amino group of adenine reduce the extent of binding-induced conformational change of the ATPase, with particularly strong effects observed for the latter two. This demonstrates the structural sensitivity of the nucleotide-ATPase complex to individual interactions between nucleotide and ATPase. All groups studied are important for binding and interactions of a given ligand group with the ATPase depend on interactions of other ligand groups. Phosphorylation of the ATPase was observed for ITP and 2'-deoxy-ATP, but not for 3'-deoxy-ATP. There is no direct link between the extent of conformational change upon nucleotide binding and the rate of phosphorylation showing that the full extent of the ATP-induced conformational change is not mandatory for phosphorylation. As observed for the nucleotide-ATPase complex, the conformation of the first phosphorylated ATPase intermediate E1PCa(2) also depends on the nucleotide, indicating that ATPase states have a less uniform conformation than previously anticipated.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
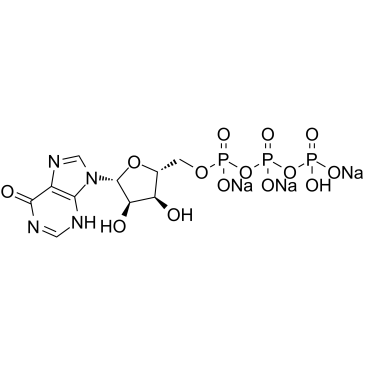 |
肌苷-5'-三磷酸三钠盐
CAS:35908-31-7 |
C10H12N4Na3O14P3 |
|
[Nucleoside-5'-triphosphate hydrolysis in the liver and kidn...
2006-01-01 [Biomed. Khim. 52 , 364-369, (2006)] |
|
Identification of an ITPase/XTPase in Escherichia coli by st...
2005-10-01 [Structure 13 , 1511-1520, (2005)] |
|
Trinitrophenyl-ATP and -ADP bind to a single nucleotide site...
1988-04-25 [J. Biol. Chem. 263 , 5569-5573, (1988)] |
|
Distinct interactions of G(salpha-long), G(salpha-short), an...
2002-08-15 [Biochem. Pharmacol. 64 , 583-593, (2002)] |
|
Characterization of an ecto-ATPase activity in Cryptococcus ...
2005-07-01 [FEMS Yeast Res. 5 , 899-907, (2005)] |