| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
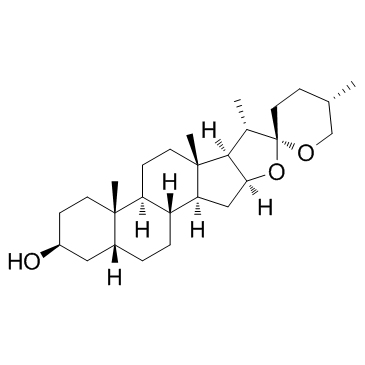 |
菝葜皂苷元; 菝葜皂甙元
CAS:126-19-2 |
|
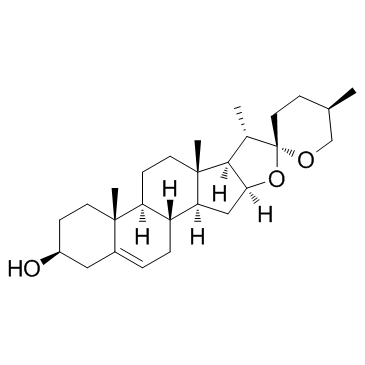 |
薯蓣皂苷元
CAS:512-04-9 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
菝葜皂苷元; 菝葜皂甙元
CAS:126-19-2 |
|
 |
薯蓣皂苷元
CAS:512-04-9 |