Deacylation reactions of 20-acetyl dinorcholanic lactones and 20,23-diacetyl furost-22-enes.
Ma Guadalupe Hernández-Linares, Jesús Sandoval-Ramírez, Socorro Meza-Reyes, Sara Montiel-Smith, María A Fernández-Herrera, Sylvain Bernès
文献索引:Steroids 75(3) , 240-4, (2010)
全文:HTML全文
摘要
We report the deacylation of (20R)-20-acetyl-23,24-dinorcholanic lactones by hydrazine hydrate, under microwave irradiation in high yields. The elimination of the 20-acetyl group proceeded with retention of configuration which contrast with other proved deacylation methods that yield a mixture of diastereoisomers. In this way, unnatural (20R)-23,24-dinorcholanic lactones can be produced rapidly on a large scale. Both (20R)- and (20S)-lactones were prepared starting from diosgenin, hecogenin and sarsasapogenin, in 72-80% overall yields.Copyright 2009 Elsevier Inc. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
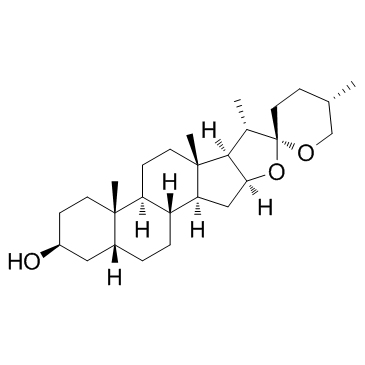 |
菝葜皂苷元; 菝葜皂甙元
CAS:126-19-2 |
C27H44O3 |
|
Regioselective cleavage of 22-oxo-23-spiroketals. Novel chol...
2012-04-01 [Steroids 77(5) , 534-41, (2012)] |
|
Sarsasapogenin induces apoptosis via the reactive oxygen spe...
2013-11-15 [Biochem. Biophys. Res. Commun. 441(2) , 519-24, (2013)] |
|
Antidepressant activity of sarsasapogenin from Anemarrhena a...
2007-01-01 [Pharmazie 62(1) , 78-9, (2007)] |
|
Diacetoxyiodobenzene-mediated synthesis of unnatural furospi...
2013-09-01 [Steroids 78(9) , 798-802, (2013)] |
|
Inhibitory effects of steroidal timosaponins isolated from t...
2010-09-01 [Immunopharmacol. Immunotoxicol. 32(3) , 357-63, (2010)] |