Conversion of proteins to diazeniumdiolate-based nitric oxide donors.
J A Hrabie, J E Saavedra, P P Roller, G J Southan, L K Keefer
文献索引:Bioconjug. Chem. 10 , 838-842, (1999)
全文:HTML全文
摘要
Michael reaction of the methoxymethyl-protected monodiazeniumdiolate of piperazine (MOM-PIPERAZI/NO) with 4-maleimidobutyric acid followed by its conversion to the N-hydroxy-succinimido ester produces a reagent capable of transferring the nitric oxide (NO)-donating diazeniumdiolate group to the terminal amines of the lysine residues contained in proteins. The reagent has been used to produce diazeniumdiolated bovine serum albumin (D-BSA) and diazeniumdiolated human serum albumin (D-HSA) containing 22 and 19 modified lysyl groups, respectively. Upon dissolution in pH 7.4 phosphate buffer at 37 degrees C, these albumin derivatives gradually released all of their contained NO (approximately 40 mol/mol of protein) with initial rates of about 30-40 pmol/min/mg and half-lives on the order of 3 weeks. This methodology is now available for use in exploiting the unique specific metabolic interactions of proteins to target NO therapy to specific physiological processes in vivo.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
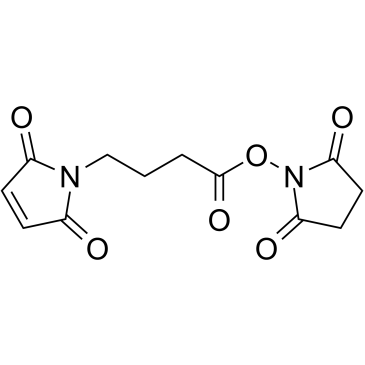 |
N-(4-马来酰亚胺丁酰基)琥珀酰亚胺
CAS:80307-12-6 |
C12H12N2O6 |
|
Highly efficient capture and harvest of circulating tumor ce...
2015-04-01 [Biomed. Microdevices 17(2) , 39, (2015)] |
|
A chitosan coated monolith for nucleic acid capture in a the...
2014-07-01 [Biomicrofluidics 8(4) , 044109, (2014)] |
|
Expansion of CTCs from early stage lung cancer patients usin...
2014-12-15 [Oncotarget 5(23) , 12383-97, (2015)] |
|
Investigation on the reaction conditions of Staphylococcus a...
2014-08-01 [Anticancer Res. 34(8) , 4521-7, (2014)] |
|
Comparison of antibody functionality using different immobil...
2003-10-20 [Biotechnol. Bioeng. 84(2) , 215-23, (2003)] |