| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
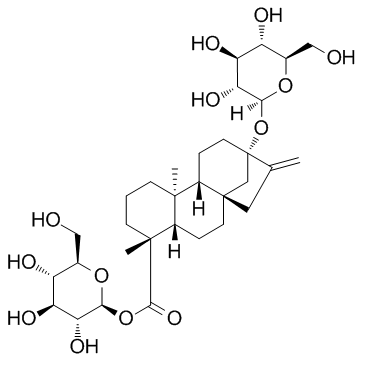 |
甜茶苷
CAS:64849-39-4 |
|
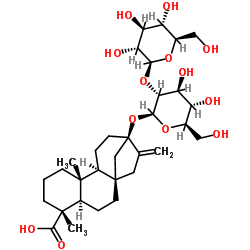 |
甜菊双糖苷
CAS:41093-60-1 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
甜茶苷
CAS:64849-39-4 |
|
 |
甜菊双糖苷
CAS:41093-60-1 |