Characterization of MobR, the 3-hydroxybenzoate-responsive transcriptional regulator for the 3-hydroxybenzoate hydroxylase gene of Comamonas testosteroni KH122-3s.
Takeshi Hiromoto, Hanako Matsue, Mariko Yoshida, Takeshi Tanaka, Hiroki Higashibata, Keiichi Hosokawa, Hiroshi Yamaguchi, Shinsuke Fujiwara
文献索引:J. Mol. Biol. 364(5) , 863-77, (2006)
全文:HTML全文
摘要
Comamonas testosteroni KH122-3s is an aerobic soil bacterium that utilizes 3-hydroxybenzoate as a sole carbon and energy source. In this strain, 3-hydroxybenzoate hydroxylase (MobA) acts on the initial step of the degradation to produce 3,4-dihydroxybenzoate, which is subsequently subjected to the meta-cleavage pathway leading to tricarboxylic acid cycle intermediates. Gene walking analysis of the upstream region of mobA revealed an open reading frame (mobR) that encodes a transcriptional regulator of the MarR family. Here, we report that MobR negatively regulates the expression of mobA, and that the repression is relieved by binding of 3-hydroxybenzoate, the substrate for MobA. A primer extension experiment was performed to determine the transcription start site for mobA and identified it at 83 bp upstream of the mobA start codon, accompanied by a typical sigma70-type promoter. The mobR gene was expressed in Escherichia coli cells and the recombinant product was purified to homogeneity. Gel mobility-shift assays and DNase I footprinting analyses indicated that MobR binds as a homodimer to an imperfect inverted repeat within the mobA-mobR intergenic region, with an apparent dissociation constant of 11.5(+/- 0.5) nM. The operator site is located between the start codon and the promoter region for mobA, suggesting that MobR functions as a transcriptional repressor for mobA expression. The results of effector-binding assays indicated that MobR, but not its isomers 4-hydroxybenzoate and salicylate, is released from the operator site by the addition of 3-hydroxybenzoate. This dissociation process is highly cooperative, with a Hill coefficient of approximately 2. In addition, CD spectroscopic studies demonstrated that MobR adopts two conformational states corresponding to the effector-bound and unbound forms. These results suggest that the MobR dimer possesses at least two effector-binding sites, and that the effector binding to MobR induces an allosteric conformational change required for dissociation of the protein-DNA complex.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
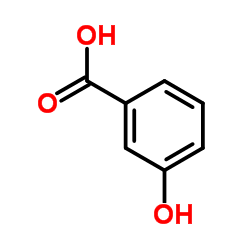 |
3-羟基苯甲酸
CAS:99-06-9 |
C7H6O3 | |
 |
P-HYDROXYBENZOATE HYDROXYLASE
CAS:9059-23-8 |
|
Degradation of anti-inflammatory drug ketoprofen by electro-...
2014-01-01 [Environ. Sci. Pollut. Res. Int. 21(14) , 8406-16, (2014)] |
|
Antagonistic control of a dual-input mammalian gene switch b...
2014-08-01 [Nucleic Acids Res. 42(14) , e116, (2014)] |
|
DNA-Linked Enzyme-Coupled Assay for Probing Glucosyltransfer...
2015-07-17 [ACS Synth. Biol. 4 , 833-41, (2015)] |
|
[3-hydroxybenzoate and 2,5-dihydroxybenzoate metabolism in R...
2012-01-01 [Mikrobiologiia 81(3) , 325-31, (2012)] |
|
Direct analysis of salicylic acid, salicyl acyl glucuronide,...
1996-01-12 [J. Chromatogr. B, Biomed. Appl. 675(1) , 61-70, (1996)] |