| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
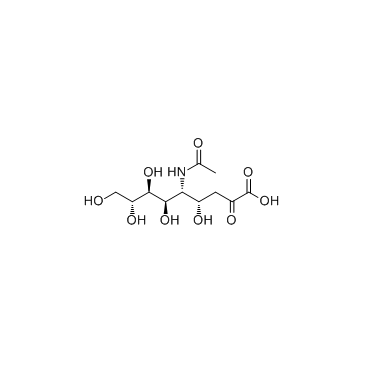 |
N-乙酰神经氨酸
CAS:131-48-6 |
|
 |
COMPLEMENT FACTOR H 来源于人类血浆
CAS:80295-65-4 |