Rapid quantification of levosulpiride in human plasma using RP-HPLC-MS/MS for pharmacokinetic and bioequivalence study.
Jin-Hee Park, Yoo-Sin Park, Si-Youn Rhim, Hyun-Jin Kim, Ok-Hwa Jhee, Yun-Sik Lee, Min-Ho Lee, Leslie M Shaw, Ju-Seop Kang
文献索引:Biomed. Chromatogr. 23(12) , 1350-6, (2009)
全文:HTML全文
摘要
A rapid and validated method for analysis of levosulpiride in human plasma using liquid chromatography coupled to tandem mass spectrometry was developed. Levosulpiride and tiapride (IS, internal standard) were extracted from alkalized plasma samples with ethylacetate and separation by RP-HPLC. Detection was performed by positive ion electrospray ionization in multiple-reaction monitoring mode, monitoring the transitions m/z 342.1 --> m/z 112.2 and m/z 329.1 --> m/z 213.2, for quantification of levosulpiride and IS, respectively. The standard calibration curves showed good linearity within the range of 2-200 ng/mL (r(2) > or = 0.9990). The lower limit of quantitation was 2 ng/mL. The retention times of levosulpiride (0.63 min) and IS (0.66 min) presented a significant time saving benefit of the proposed method. No significant metabolic compounds were found to interfere with the analysis. This method offered good precision and accuracy and was successfully applied for the pharmacokinetic and bioequivalence study of a 25 mg of levosulpiride tablet in 24 healthy Korean volunteers.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
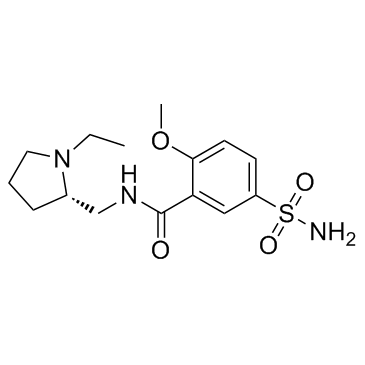 |
(S)-(-)-舒必利
CAS:23672-07-3 |
C15H23N3O4S |
|
Chemical genetics reveals a complex functional ground state ...
2007-05-01 [Nat. Chem. Biol. 3(5) , 268-273, (2007)] |
|
The use of dried blood spots for quantification of 15 antips...
2015-06-01 [Drug Test. Anal. 7 , 502-11, (2015)] |
|
5-HT2A receptor activation is necessary for CO2-induced arou...
2015-07-01 [J. Neurophysiol. 114 , 233-43, (2015)] |
|
Liquid chromatography-tandem mass spectrometry quantificatio...
2010-08-15 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 878(24) , 2280-5, (2010)] |
|
Influence of ABCB1 genetic polymorphisms on the pharmacokine...
2010-08-11 [Neuroscience 169(1) , 378-87, (2010)] |