Liquid chromatography-tandem mass spectrometry quantification of levosulpiride in human plasma and its application to bioequivalence study.
Prasad B Phapale, Hae Won Lee, Mi-Sun Lim, Sook Jin Seong, Eun-Hee Kim, Jeonghyeon Park, Miran Lee, Sung-Kyu Hwang, Young-Ran Yoon
文献索引:J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 878(24) , 2280-5, (2010)
全文:HTML全文
摘要
An improved method for determining levels of levosulpiride in human plasma using ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) was developed and validated. The protein precipitation method was used for plasma sample preparation. Levosulpiride and an internal standard (IS) were isocratically separated on a UPLC BEH C(18) column with a mobile phase of ammonium formate buffer (1mM, adjusted to pH 3 with formic acid) and acetonitrile (60:40, v/v). MS/MS detection was performed by monitoring the parent-->daughter pair of levosulpiride and the IS at m/z 342-->112 and 329-->256, respectively. The method was linear from 2.5 to 200ng/mL and exhibited acceptable precision and percent recovery. The method was successfully demonstrated in pharmacokinetic and bioequivalence studies of two levosulpiride oral formulations administered to healthy volunteers. When compared to the previous LC-MS methods, the proposed method is faster, well-validated, and uses lesser plasma volume and a similar sensitivity. The use of UPLC allowed rapid and sensitive quantification of levosulpiride, making this method suitable for high-throughput clinical applications.Copyright 2010 Elsevier B.V. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
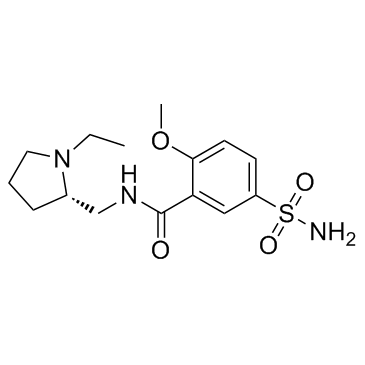 |
(S)-(-)-舒必利
CAS:23672-07-3 |
C15H23N3O4S |
|
Chemical genetics reveals a complex functional ground state ...
2007-05-01 [Nat. Chem. Biol. 3(5) , 268-273, (2007)] |
|
The use of dried blood spots for quantification of 15 antips...
2015-06-01 [Drug Test. Anal. 7 , 502-11, (2015)] |
|
5-HT2A receptor activation is necessary for CO2-induced arou...
2015-07-01 [J. Neurophysiol. 114 , 233-43, (2015)] |
|
Influence of ABCB1 genetic polymorphisms on the pharmacokine...
2010-08-11 [Neuroscience 169(1) , 378-87, (2010)] |
|
Diabetes mellitus and drug-induced Parkinsonism: a case-cont...
2009-09-15 [J. Neurol. Sci. 284(1-2) , 140-3, (2009)] |