| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
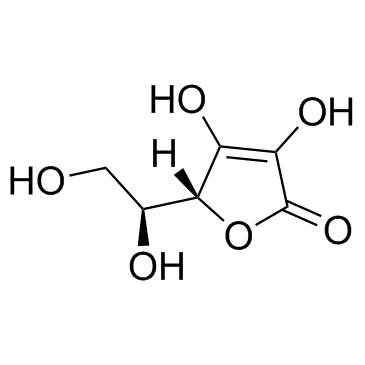 |
抗坏血酸
CAS:50-81-7 |
|
 |
透明质酸酶
CAS:37259-53-3 |
|
 |
L-抗坏血酸棕榈酸酯
CAS:137-66-6 |