| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
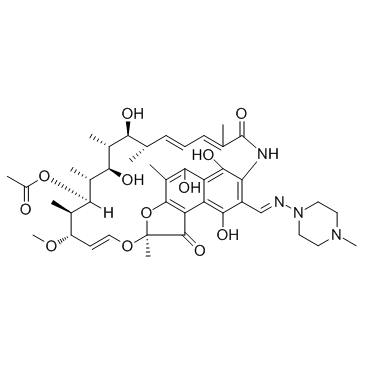 |
利福平
CAS:13292-46-1 |
|
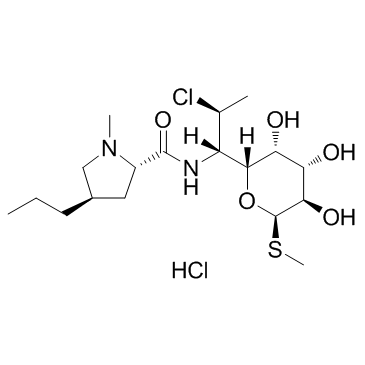 |
盐酸克林霉素
CAS:21462-39-5 |
|
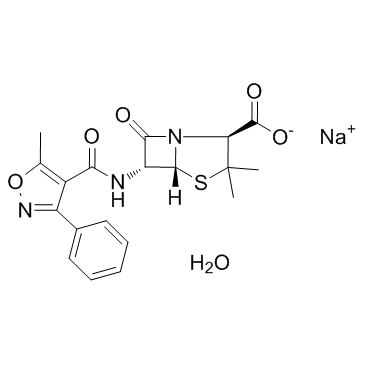 |
苯唑西林钠
CAS:7240-38-2 |