| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
丙泊酚
CAS:2078-54-8 |
|
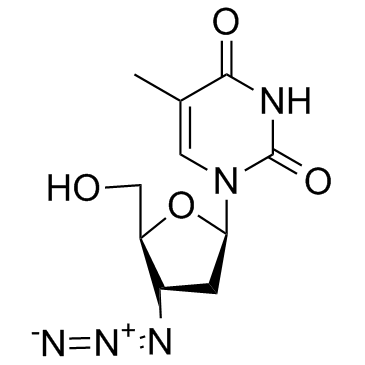 |
齐多夫定
CAS:30516-87-1 |
|
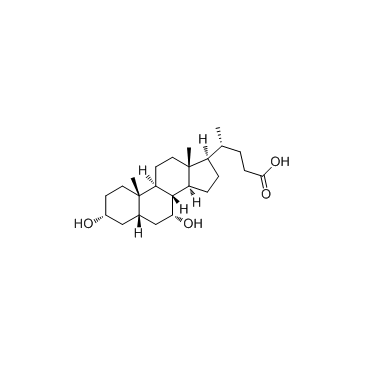 |
鹅去氧胆酸; 鹅脱氧胆酸
CAS:474-25-9 |
|
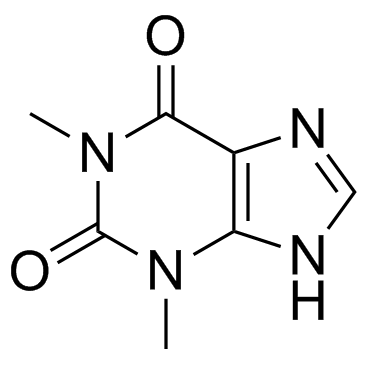 |
茶碱
CAS:58-55-9 |
|
 |
非那雄胺
CAS:98319-26-7 |
|
 |
血清胺盐酸盐
CAS:153-98-0 |
|
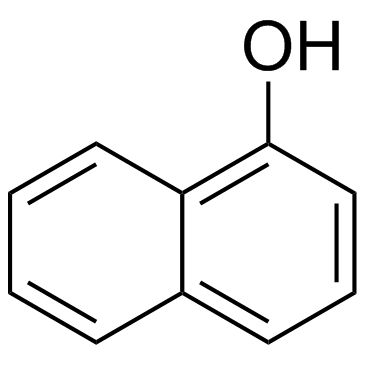 |
1-萘酚
CAS:90-15-3 |
|
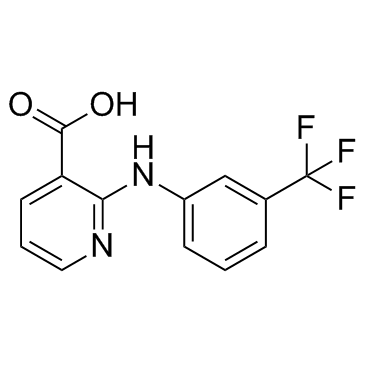 |
氟尼酸
CAS:4394-00-7 |
|
 |
依法韦仑
CAS:154598-52-4 |
|
 |
盐酸三氟拉嗪
CAS:440-17-5 |