| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
甲醛
CAS:50-00-0 |
|
 |
L-赖氨酸盐酸盐
CAS:657-27-2 |
|
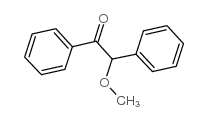 |
安息香甲醚
CAS:3524-62-7 |
|
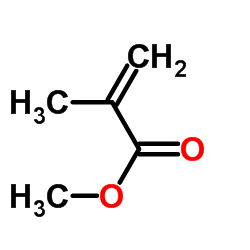 |
甲基丙烯酸甲酯
CAS:80-62-6 |
|
 |
邻苯二甲酸单丁酯
CAS:131-70-4 |
|
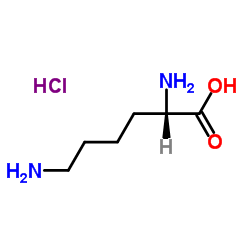 |
盐酸赖氨酸
CAS:10098-89-2 |
|
 |
4,4'-(1-甲基亚乙基)双苯酚与氯甲基环氧乙烷和Α-氢基-Ω-羟基聚(氧化-1,2-乙二基)的聚合物
CAS:42617-82-3 |