| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
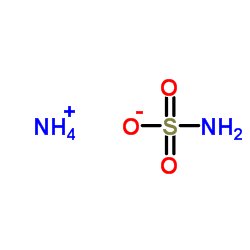 |
氨基磺酸铵
CAS:7773-06-0 |
|
![双(二氧化硫)-1,4-二氮杂双环[2.2.2]辛烷加合物 结构式](https://image.chemsrc.com/caspic/393/119752-83-9.png) |
双(二氧化硫)-1,4-二氮杂双环[2.2.2]辛烷加合物
CAS:119752-83-9 |
|
 |
氨基磺酸
CAS:5329-14-6 |