| Structure | Name/CAS No. | Articles |
|---|---|---|
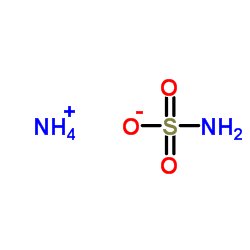 |
Ammonium sulfamate
CAS:7773-06-0 |
|
![1,4-Diazoniabicyclo[2.2.2]octane-1,4-disulfinate Structure](https://image.chemsrc.com/caspic/393/119752-83-9.png) |
1,4-Diazoniabicyclo[2.2.2]octane-1,4-disulfinate
CAS:119752-83-9 |
|
 |
Sulfamic acid
CAS:5329-14-6 |