| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
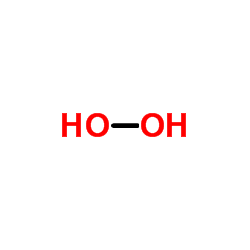 |
过氧化氢
CAS:7722-84-1 |
|
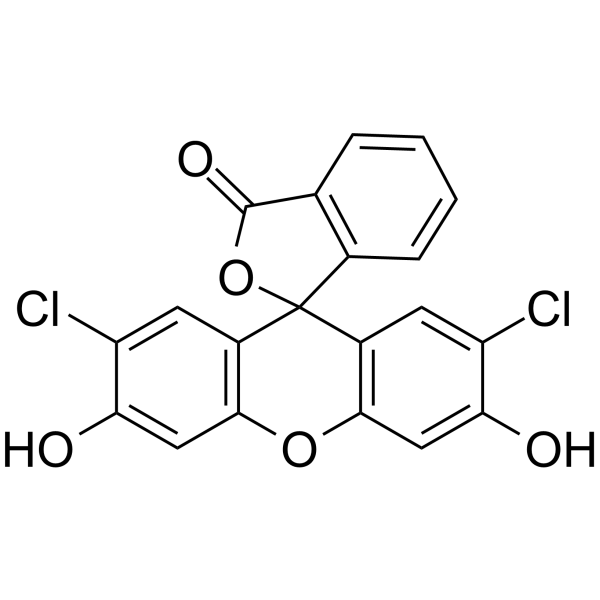 |
2',7'-二氯荧光素
CAS:76-54-0 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
过氧化氢
CAS:7722-84-1 |
|
 |
2',7'-二氯荧光素
CAS:76-54-0 |