Novel and highly regioselective route for synthesis of 5-fluorouridine lipophilic ester derivatives by lipozyme TL IM.
Huai Wang, Min-Hua Zong, Hong Wu, Wen-Yong Lou
文献索引:J. Biotechnol. 129(4) , 689-95, (2007)
全文:HTML全文
摘要
For the first time, lipozyme TL IM, an inexpensive lipase from Thermomyces lanuginosa, was successfully applied to the regioselective synthesis of lipophilic 5-fluorouridine ester derivatives. The ESI-MS and (13)C NMR analysis confirmed that the end products of the acylation were 5'-O-acyl 5-fluorouridines, more powerful anti-tumor drugs than 5-fluorouridine itself. Notably, the chain length of acyl donors had an obvious effect on the initial rate and the maximum substrate conversion of the regioselective acylation. The acylation of 5-fluorouridine with vinyl laurate was used as a model to explore the influence of various factors on the reaction with respect to the initial rate, the maximum substrate conversion and the regioselectivity. The optimum water activity, the molar ratio of vinyl laurate to 5-fluorouridine, reaction temperature and shaking rate were 0.07, 15/1, 45 degrees C and 200rpm, respectively, under which the maximum substrate conversion and the regioselectivity were as high as 98.4 and >99%, respectively, after a reaction time of around 6h.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
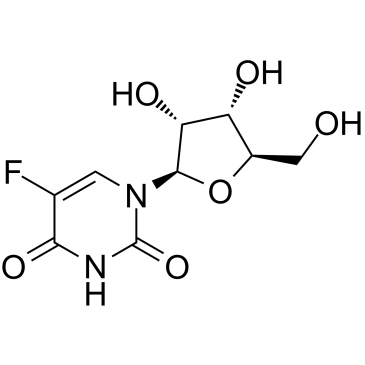 |
5-氟尿嘧啶核苷
CAS:316-46-1 |
C9H11FN2O6 |
|
Development of an LC-MS/MS assay for the quantitative determ...
2015-06-10 [J. Pharm. Biomed. Anal. 110 , 58-66, (2015)] |
|
N4-[Alkyl-(hydroxyphosphono)phosphonate]-cytidine—New drugs ...
2011-01-01 [Bioorg. Med. Chem. 19(11) , 3520-6, (2011)] |
|
Evidence for Transcriptional Activity in the Syncytiotrophob...
2009-04-01 [Placenta 30(4) , 329-34, (2009)] |
|
Plastid uridine salvage activity is required for photoassimi...
2011-08-01 [Plant Cell 23(8) , 2991-3006, (2011)] |
|
TDP-43 localizes in mRNA transcription and processing sites ...
2009-09-01 [J. Struct. Biol. 167(3) , 235-41, (2009)] |