| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
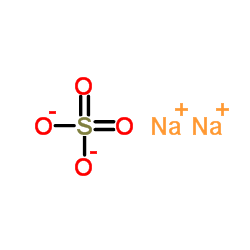 |
元明粉
CAS:7757-82-6 |
|
 |
结晶硫酸钠,十水合物
CAS:7727-73-3 |
|
 |
钡
CAS:7440-39-3 |
|
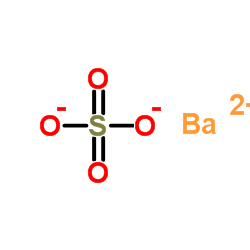 |
硫酸钡
CAS:7727-43-7 |
|
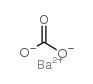 |
碳酸钡
CAS:513-77-9 |
|
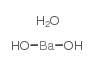 |
一水合氢氧化钡
CAS:22326-55-2 |
|
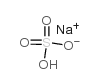 |
硫酸氢钠
CAS:7681-38-1 |
|
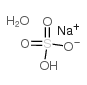 |
硫酸氢钠,一水
CAS:10034-88-5 |
|
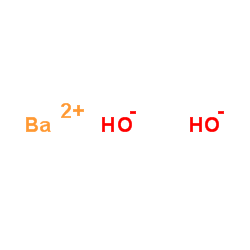 |
氢氧化钡
CAS:17194-00-2 |
|
 |
氢氧化钡,八水合物
CAS:12230-71-6 |