| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
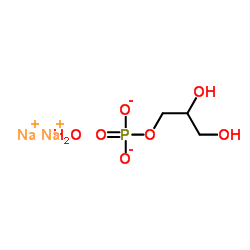 |
甘油磷酸钠
CAS:154804-51-0 |
|
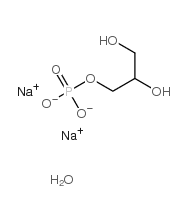 |
甘油磷酸二钠盐
CAS:55073-41-1 |
|
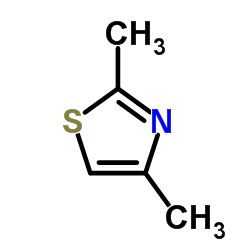 |
2,4-二甲基噻唑
CAS:541-58-2 |