| Structure | Name/CAS No. | Articles |
|---|---|---|
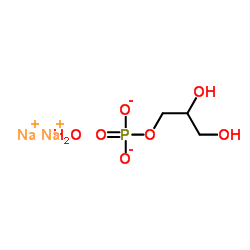 |
Sodium glycerophosphate
CAS:154804-51-0 |
|
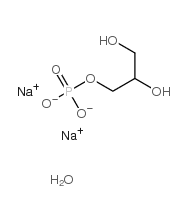 |
Glycerol phosphate disodium salt hydrate
CAS:55073-41-1 |
|
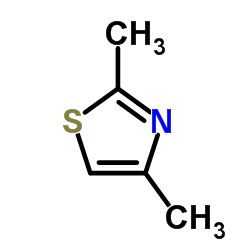 |
2.4-Dimethyl thiazole
CAS:541-58-2 |