| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
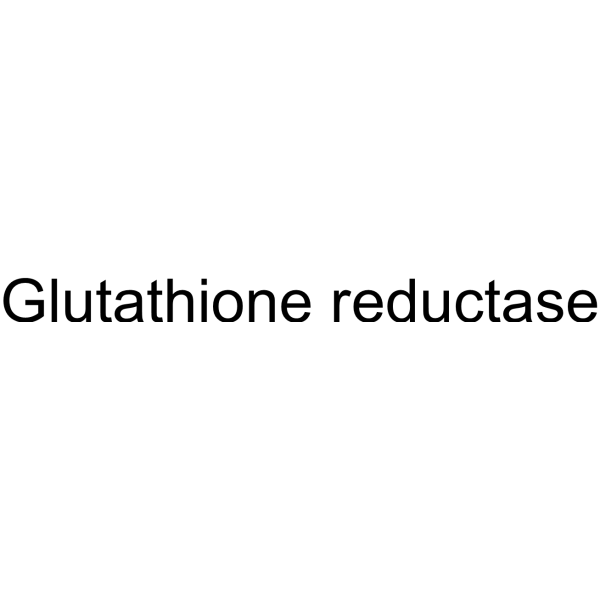 |
谷胱甘肽还原酶(来源于酿酒酵母)
CAS:9001-48-3 |
|
 |
硫氧还蛋白 来源于大肠杆菌
CAS:52500-60-4 |