| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
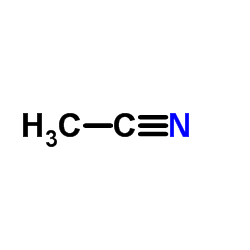 |
乙腈
CAS:75-05-8 |
|
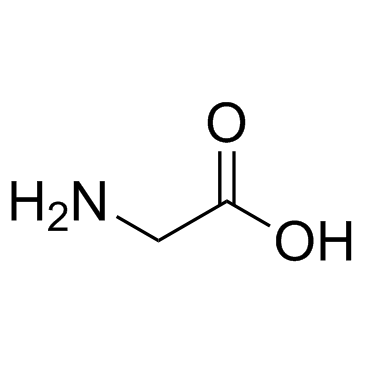 |
甘氨酸
CAS:56-40-6 |
|
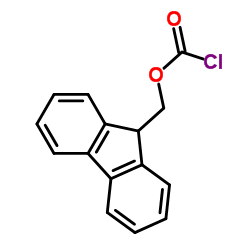 |
芴甲氧羰酰氯(Fmoc-Cl)
CAS:28920-43-6 |