| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
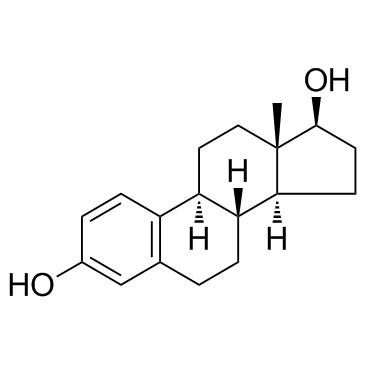 |
雌二醇
CAS:50-28-2 |
|
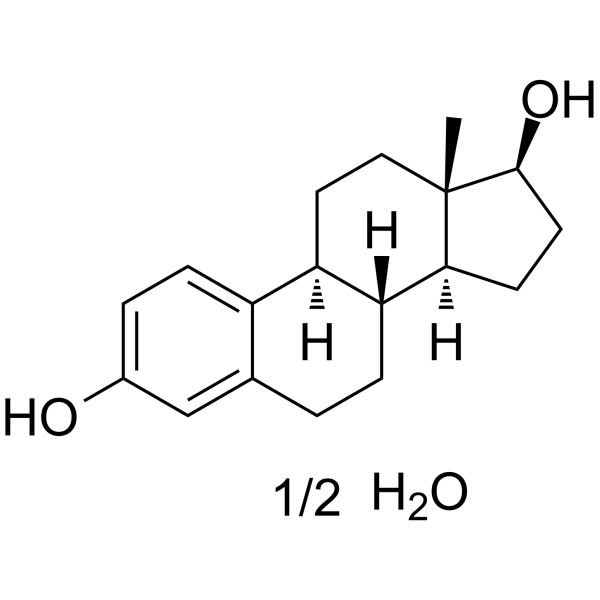 |
beta-雌二醇半水合物
CAS:35380-71-3 |
|
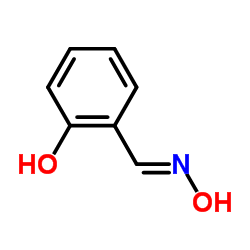 |
水杨醛肟
CAS:94-67-7 |