| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
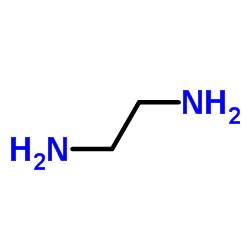 |
乙二胺
CAS:107-15-3 |
|
 |
乙腈
CAS:75-05-8 |
|
 |
甲酸
CAS:64-18-6 |
|
 |
甲醇
CAS:67-56-1 |
|
 |
三氟乙酸(TFA)
CAS:76-05-1 |
|
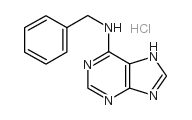 |
6-苄氨基嘌呤 盐酸盐
CAS:162714-86-5 |
|
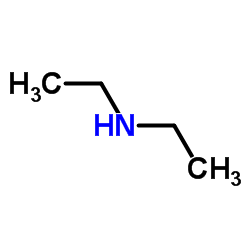 |
二乙胺
CAS:109-89-7 |
|
 |
盐酸西替利嗪
CAS:83881-52-1 |
|
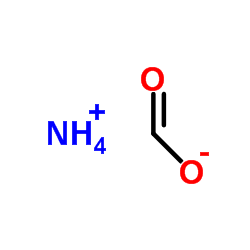 |
甲酸铵
CAS:540-69-2 |
|
 |
盐酸左旋西替利嗪
CAS:130018-87-0 |