| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
丙酮
CAS:67-64-1 |
|
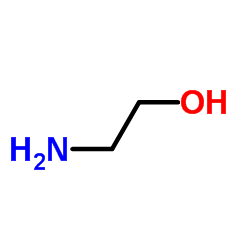 |
2-氨基乙醇
CAS:141-43-5 |
|
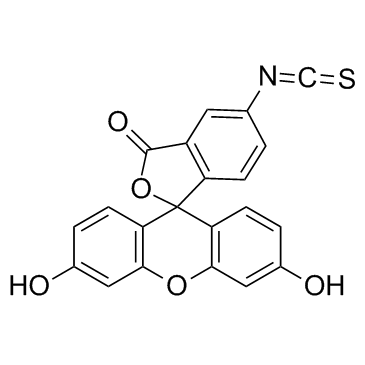 |
异硫氰酸荧光素酯
CAS:3326-32-7 |
|
 |
甲醇
CAS:67-56-1 |
|
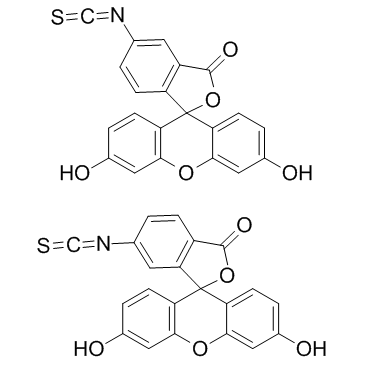 |
异硫氰酸荧光素
CAS:27072-45-3 |
|
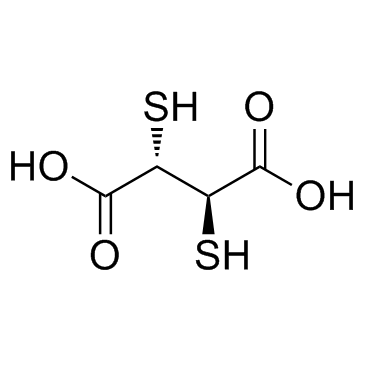 |
2,3-二巯基丁二酸
CAS:304-55-2 |
|
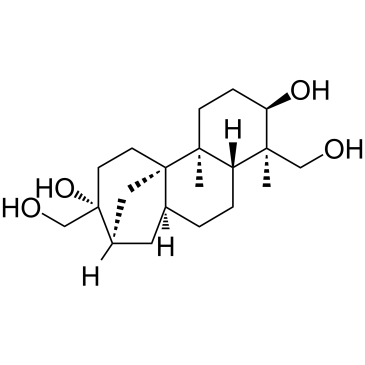 |
阿非科林
CAS:38966-21-1 |
|
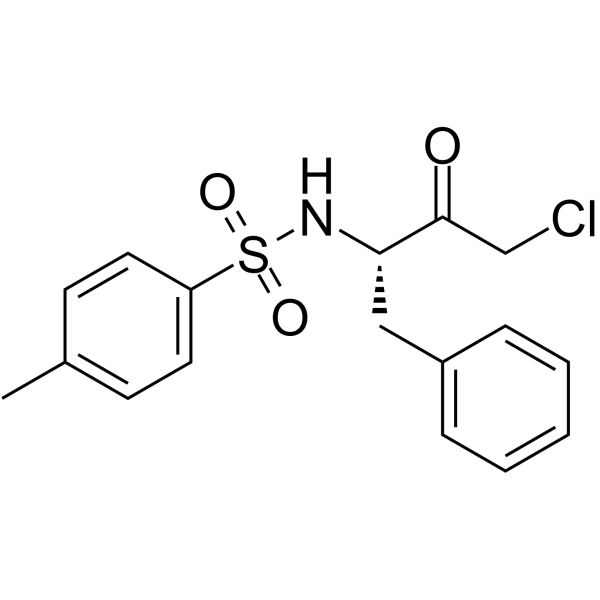 |
N-(对甲苯磺酰基)-L-苯丙氨酰甲基氯酮(TPCK)
CAS:402-71-1 |
|
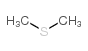 |
二甲基硫
CAS:75-18-3 |
|
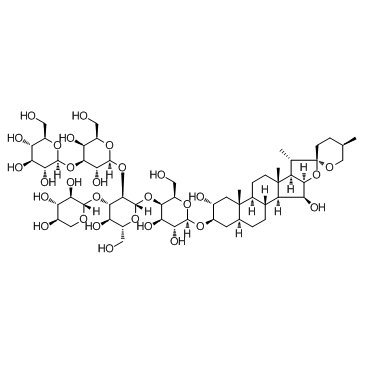 |
毛地黄皂苷
CAS:11024-24-1 |