Understanding the effect of different assay formats on agonist parameters: a study using the µ-opioid receptor.
Sarah A Nickolls, Alison Waterfield, Rachael E Williams, Ross A Kinloch
文献索引:J. Biomol. Screen. 16(7) , 706-16, (2011)
全文:HTML全文
摘要
The correct interpretation of data is fundamental to the study of G-protein-coupled receptor pharmacology. Often, new assay technologies are assimilated into the drug discovery environment without full consideration of the data generated. In this study, the authors look at µ-opioid receptor agonists in three different assays: (1) [(35)S]GTPγS binding, (2) inhibition of forskolin-stimulated cAMP production, and (3) β-arrestin recruitment. Agonist-concentration effect curves were performed before and after treatment with the irreversible antagonist β-funaltrexamine, and where appropriate, these data were fitted to the operational model of agonism. The Z' value was highest in the β-arrestin assay, followed by the [(35)S]GTPγS and cAMP assays. The cAMP data fitted well to the operational model, as did the [(35)S]GTPγS data, but the [(35)S]GTPγS assay led to an apparent overestimation of K(A) values. However, in the β-arrestin assay, data did not fit the operational model, as treatment with β-funaltrexamine reduced the Emax proportionally to receptor number, with no change in EC(50). In addition, the EC(50) values generated correlated well with affinity values. In conclusion, the β-arrestin recruitment assay does not fit with traditional pharmacological theory but is of great utility as the EC(50) value generated is a good approximation of affinity.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
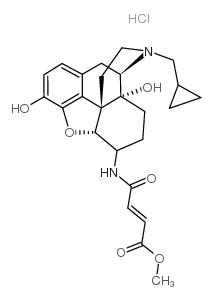 |
β-富纳曲胺盐酸盐
CAS:72786-10-8 |
C25H31ClN2O6 |
|
Novel orally available salvinorin A analog PR-38 inhibits ga...
2014-07-01 [J. Pharmacol. Exp. Ther. 350(1) , 69-78, (2014)] |
|
Activation of the cloned human kappa opioid receptor by agon...
1997-08-01 [J. Pharmacol. Exp. Ther. 282 , 676, (1997)] |
|
Activation of phosphatidylinositol 3-kinase/Akt-mammalian ta...
2010-11-24 [Neuroscience 171(1) , 134-43, (2010)] |
|
The mu-opioid receptor-selective peptide antagonists, antana...
2013-01-10 [Prog. Neuropsychopharmacol. Biol. Psychiatry 40 , 126-31, (2013)] |
|
The involvement of micro-opioid receptors in the central ner...
2010-01-01 [Int. Arch. Allergy Immunol. 152(4) , 342-52, (2010)] |