Cannabinoids in the treatment of chemotherapy-induced nausea and vomiting.
Barbara Todaro
文献索引:J. Natl. Compr. Canc. Netw. 10(4) , 487-92, (2012)
全文:HTML全文
摘要
Before the introduction of the serotonin receptor antagonists (5-HT3 receptor antagonists) in the early 1990s, limited effective options were available to prevent and treat chemotherapy-induced nausea and vomiting (CINV). In 1985, the FDA approved 2 cannabinoid derivatives, dronabinol and nabilone, for the treatment of CINV not effectively treated by other agents. Today, the standard of care for prevention of CINV for highly and moderately emetogenic chemotherapy is a 5-HT3 receptor antagonist, dexamethasone, with or without aprepitant or fosaprepitant. With the approval of safer and more effective agents, cannabinoids are not recommended as first-line treatment for the prevention of CINV and are reserved for patients with breakthrough nausea and vomiting. Because of medical and legal concerns, the use of marijuana is not recommended for management of CINV and is not part of the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Antiemesis. Although patients may like to pursue this treatment option in states that have approved the use of marijuana for medical purposes, its use remains legally and therapeutically controversial.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
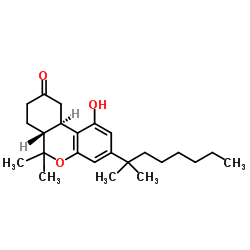 |
大麻隆
CAS:51022-71-0 |
C24H36O3 |
|
Oral nabilone capsules in the treatment of chemotherapy-indu...
2008-01-01 [Expert Opin. Investig. Drugs 17(1) , 85-95, (2008)] |
|
A randomized, double-blinded, crossover pilot study assessin...
2010-05-01 [Arch. Phys. Med. Rehabil. 91(5) , 703-7, (2010)] |
|
An open-label comparison of nabilone and gabapentin as adjuv...
2011-01-01 [Pain Pract. 11(4) , 353-68, (2011)] |
|
Subjective, cognitive and cardiovascular dose-effect profile...
2013-09-01 [Addict. Biol. 18(5) , 872-81, (2013)] |
|
Analgesic and antihyperalgesic effects of nabilone on experi...
2008-04-01 [Curr. Med. Res. Opin. 24(4) , 1017-24, (2008)] |