| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
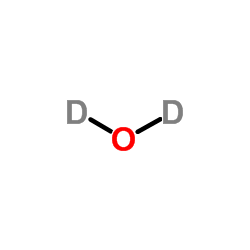 |
重水
CAS:7789-20-0 |
|
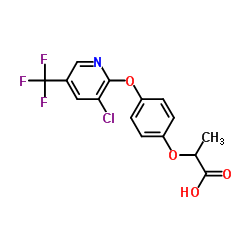 |
吡氟氯禾灵
CAS:69806-34-4 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
重水
CAS:7789-20-0 |
|
 |
吡氟氯禾灵
CAS:69806-34-4 |