| Structure | Name/CAS No. | Articles |
|---|---|---|
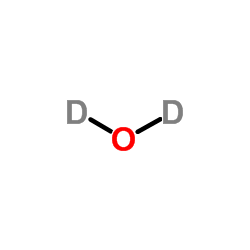 |
Heavy water
CAS:7789-20-0 |
|
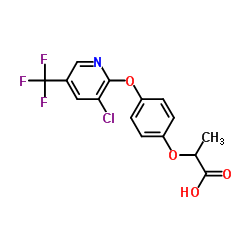 |
haloxyfop
CAS:69806-34-4 |