| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
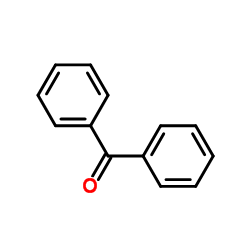 |
二苯甲酮
CAS:119-61-9 |
|
 |
组织蛋白酶 L
CAS:60616-82-2 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
二苯甲酮
CAS:119-61-9 |
|
 |
组织蛋白酶 L
CAS:60616-82-2 |