| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
甲酸
CAS:64-18-6 |
|
 |
氯化钠
CAS:7647-14-5 |
|
 |
乙醇
CAS:64-17-5 |
|
 |
甲醇
CAS:67-56-1 |
|
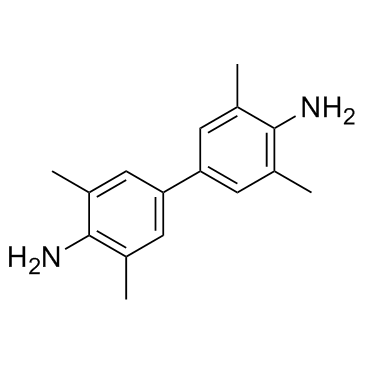 |
3,3′,5,5′-四甲基联苯胺(TMB)
CAS:54827-17-7 |
|
 |
六氢吡啶
CAS:110-89-4 |
|
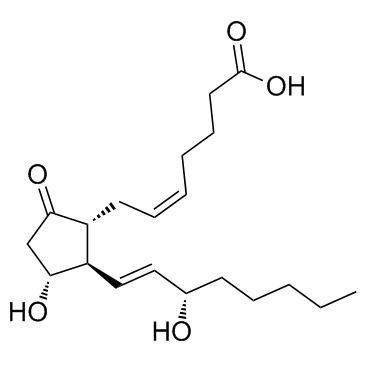 |
地诺前列酮
CAS:363-24-6 |
|
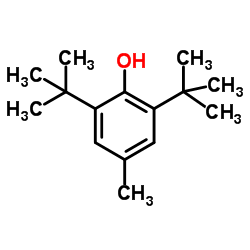 |
抗氧剂BHT
CAS:128-37-0 |
|
 |
罗格列酮
CAS:122320-73-4 |
|
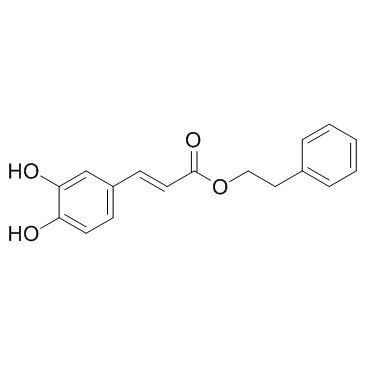 |
咖啡酸苯乙酯
CAS:104594-70-9 |