| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
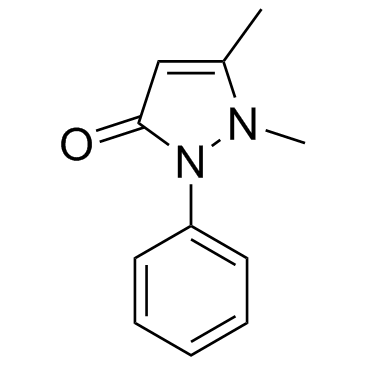 |
安替比林
CAS:60-80-0 |
|
 |
酮康唑
CAS:65277-42-1 |
|
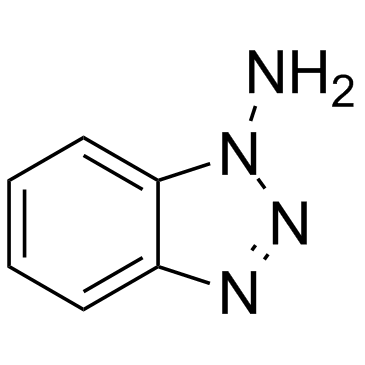 |
1-氨基苯并三唑
CAS:1614-12-6 |
|
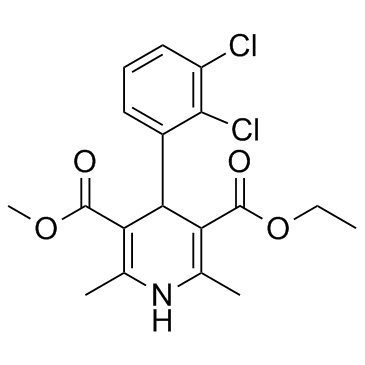 |
非洛地平
CAS:72509-76-3 |