| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
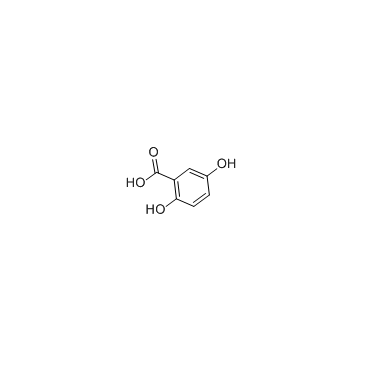 |
2,5-二羟基苯甲酸
CAS:490-79-9 |
|
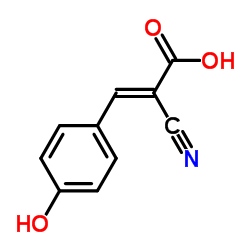 |
α-氰基-4-羟基肉桂酸
CAS:28166-41-8 |
|
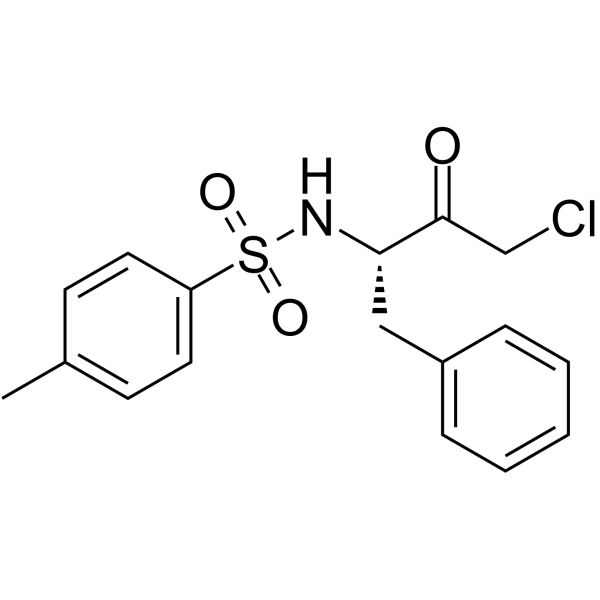 |
N-(对甲苯磺酰基)-L-苯丙氨酰甲基氯酮(TPCK)
CAS:402-71-1 |
|
 |
5-甲氧基水杨酸
CAS:2612-02-4 |