Analysis of biological thiols: derivatization with monobromotrimethylammoniobimane and characterization by electrophoresis and chromatography.
R C Fahey, G L Newton, R Dorian, E M Kosower, Robert C. Fahey, Gerald L. Newton, Randel Dorian, Edward M. Kosower
文献索引:Anal. Biochem. 107(1) , 1-10, (1980)
全文:HTML全文
摘要
A new method for analysis of biological thiols based upon their conversion to fluorescent derivatives by reaction with monobromotrimethylammoniobimane (qBBr) is described. The derivatives are separated by chromatography and by electrophoresis on cellulose thinlayer chromatography plates. The use of two-dimensional mapping makes it possible to differentiate between a wide variety of biological thiols including N-acetylcysteine, CoA, cysteine, cysteinylglycine, cysteamine, ergothioneine, glutathione, γ-glutamylcysteine, homocysteine, mercaptopyrimidine, pantetheine, 4′-phosphopanetheine, thiosulfate, and thiouracil. For applications to biological samples thiols were isolated from crude extracts by binding to a mercuriagarose gel. Following removal from the gel with dithiothreitol, the thiols were derivatized with qBBr. The methods were tested by showing that glutathione is the major thiol in human red blood cells, that glutathione and ergothioneine are the major thiols in Neurospora crassa conidia, and that Bacillus cereus vegetative cells lack glutathione but contain cysteine, pantetheine, and an unidentified thiol in significant amounts.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
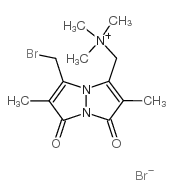 |
单溴(三甲基铵)二溴化溴
CAS:71418-45-6 |
C13H19Br2N3O2 |
|
Cell surface thiols, but not intracellular glutathione, are ...
1985-10-01 [Immunol. Invest. 14(5) , 401-14, (1985)] |
|
The open/closed conformational equilibrium of aspartate amin...
1991-03-14 [Eur. J. Biochem. 196 , 329, (1991)] |
|
Thiol labeling with bromobimanes.
1987-01-01 [Meth. Enzymol. 143 , 76, (1987)] |
|
Localization of thiol and disulfide groups in guinea pig spe...
1984-11-01 [Biol. Reprod. 31(4) , 797-809, (1984)] |
|
Protein disulfide isomerase mediates integrin-dependent adhe...
2000-06-16 [FEBS Lett. 475(2) , 89-92, (2000)] |