The open/closed conformational equilibrium of aspartate aminotransferase. Studies in the crystalline state and with a fluorescent probe in solution.
D Picot, E Sandmeier, C Thaller, M G Vincent, P Christen, J N Jansonius
文献索引:Eur. J. Biochem. 196 , 329, (1991)
全文:HTML全文
摘要
Aspartate aminotransferase undergoes major shifts in the conformational equilibrium of the protein matrix during transamination. The present study defines the two conformational states of the enzyme by crystallographic analysis, examines the conditions under which the enzyme crystallizes in each of these conformations, and correlates these conditions with the conformational behaviour of the enzyme in solution, as monitored by a fluorescent reporter group. Cocrystallization of chicken mitochondrial aspartate aminotransferase with inhibitors and covalent coenzymesubstrate adducts yields three different crystal forms. Unliganded enzyme forms triclinic crystals of the open conformation, the structure of which has been solved (space group P1) [Ford, G. C., Eichele, G. & Jansonius, J. N. (1980) Proc. Natl Acad. Sci. USA 77, 2559-2563; Kirsch, J. F., Eichele, G., Ford, G. C., Vincent, M. G., Jansonius, J. N., Gehring, H. & Christen, P. (1984) J. Mol. Biol. 174, 487-525]. Complexes of the enzyme with dicarboxylate ligands form monoclinic or orthorhombic crystals of the closed conformation. The results of structure determinations of the latter two crystal forms at 0.44 nm resolution are described here. In the closed conformation, the small domain has undergone a rigid-body rotation of 12-14 which closes the active-site pocket. Shifts in the conformational equilibrium of aspartate aminotransferase in solution, as induced by substrates, substrate analogues and specific dicarboxylic inhibitors, can be monitored by changes in the relative fluoresence yield of the enzyme labelled at Cys166 with monobromotrimethylammoniobimane. The pyridoxal and pyridoxamine forms of the labelled enzyme show the same fluorescence properties, whereas in the apoenzyme the fluorescence intensity is reduced by 30%. All active-site ligands, if added to the labelled pyridoxal enzyme at saturating concentrations, cause a decrease in the fluorescence intensity by 40-70% and a blue shift of maximally 5 nm. Comparison of the fluorescence properties of the enzyme in various functional states with the crystallographic data shows that both techniques probe the same conformational equilibrium. The conformational change that closes the active site seems to be ligand-induced in the reaction of the pyridoxal form of the enzyme and syncatalytic in the reverse reaction with the pyridoxamine enzyme.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
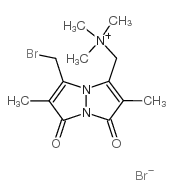 |
单溴(三甲基铵)二溴化溴
CAS:71418-45-6 |
C13H19Br2N3O2 |
|
Cell surface thiols, but not intracellular glutathione, are ...
1985-10-01 [Immunol. Invest. 14(5) , 401-14, (1985)] |
|
Thiol labeling with bromobimanes.
1987-01-01 [Meth. Enzymol. 143 , 76, (1987)] |
|
Localization of thiol and disulfide groups in guinea pig spe...
1984-11-01 [Biol. Reprod. 31(4) , 797-809, (1984)] |
|
Protein disulfide isomerase mediates integrin-dependent adhe...
2000-06-16 [FEBS Lett. 475(2) , 89-92, (2000)] |
|
Conformational changes in mitochondrial aspartate aminotrans...
1984-01-01 [Prog. Clin. Biol. Res. 144B , 117-24, (1984)] |