| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
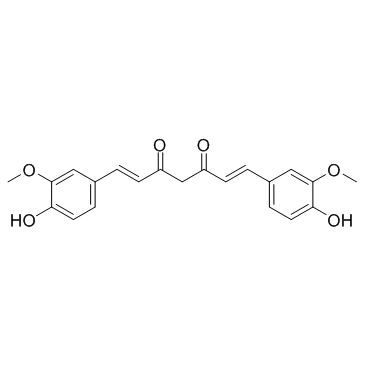 |
姜黄素
CAS:458-37-7 |
|
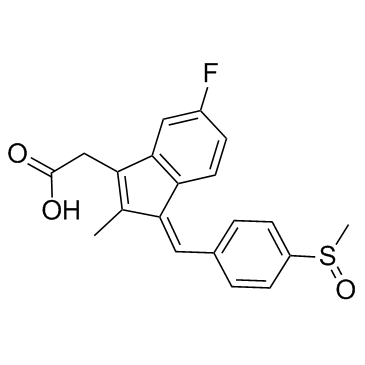 |
舒林酸
CAS:38194-50-2 |
|
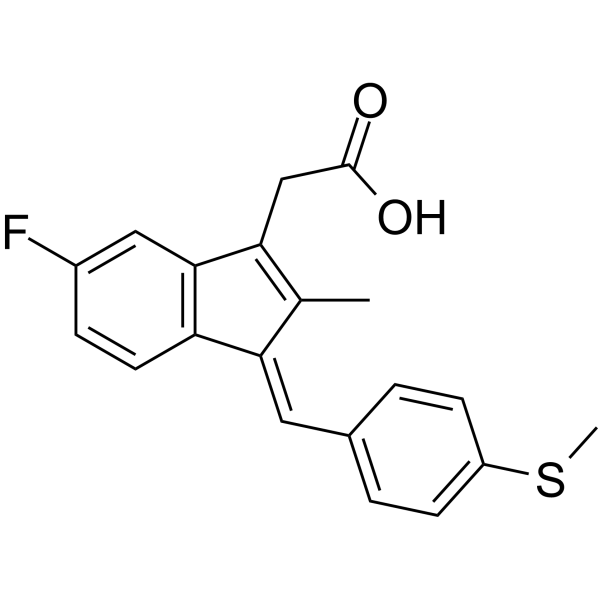 |
5-氟-2-甲基-1-(4-甲硫基亚苄基)茚-3-乙酸
CAS:32004-67-4 |
|
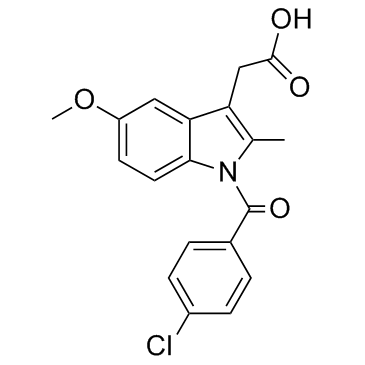 |
吲哚美辛
CAS:53-86-1 |