| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
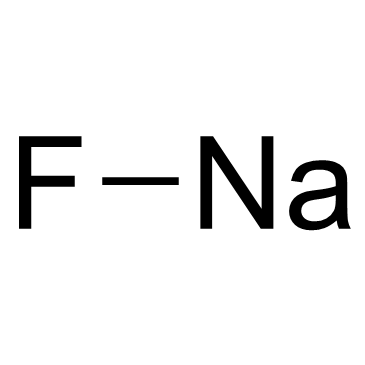 |
氟化钠
CAS:7681-49-4 |
|
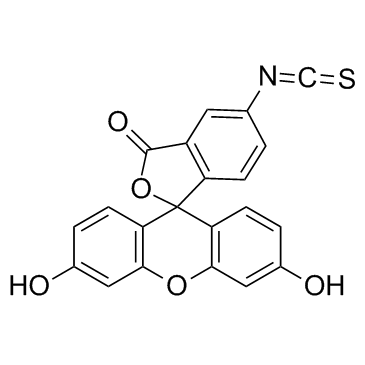 |
异硫氰酸荧光素酯
CAS:3326-32-7 |
|
 |
十二烷基硫酸钠
CAS:151-21-3 |
|
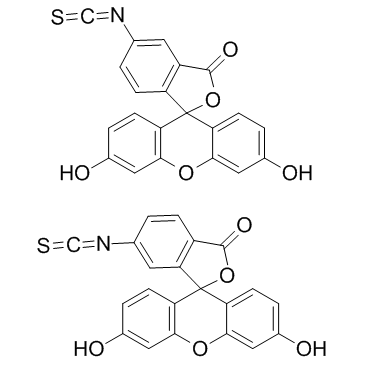 |
异硫氰酸荧光素
CAS:27072-45-3 |
|
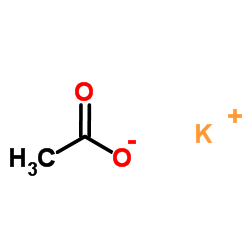 |
醋酸钾
CAS:127-08-2 |
|
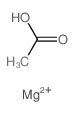 |
乙酸镁
CAS:142-72-3 |
|
 |
4-羟乙基哌嗪乙磺酸
CAS:7365-45-9 |
|
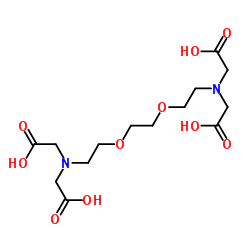 |
3,6-二氧杂-1,8-辛二胺四乙酸(EGTA)
CAS:67-42-5 |
|
 |
腺苷5'-二磷酸核糖钠盐
CAS:68414-18-6 |
|
 |
去氧胆酸钠
CAS:302-95-4 |